MENU
TH | THB
TH | THB
-
- Benchtop Centrifuges
- Floor-Standing Centrifuges
- Refrigerated Centrifuges
- Microcentrifuges
- Multipurpose Centrifuges
- High-Speed Centrifuges
- Ultracentrifuges
- Concentrator
- High-Speed and Ultracentrifugation Consumables
- Accessories
- Tubes
- Plates
- Device Management Software
- Sample and Information Management
No results found
Search Suggestions

Production of Recombinant Human Phosphoserine Phosphatase Using the Innova® S44i Shaker and the Centrifuge Harvesting Bundle 4 x 1.5 L
NATASCHA WEISS, VINCENT DUFEY, SILVIA TEJERINA VARGAS, AURÉLIE TACHENY
The production of recombinant proteins by genetically modified organisms is a fundamental biotechnology technique. The goal is to generate a high yield of functional protein which requires large-scale cell culture with good cell growth and excellent protein expression. Therefore, a robust shaker is necessary that can shake cultures at high speeds on a liter scale. Centrifugation is used to harvest the cells, to clarify the cell lysate and (if necessary) to concentrate the protein solution.
Harvesting several liters of culture is time-consuming as a result of numerous manual steps required for each individual vessel [1]. For variable culture volumes and further centrifugation steps additional equipment is needed.
In this Application Note*, the production of a recombinant protein is presented using a workflow for culturing and harvesting bacteria and purifying proteins. The aim is to demonstrate that a high yield of functional recombinant protein can be realized by using the Innova S44i shaker and a high-speed centrifuge (as a system solution consisting of Harvesting Bundle combined with the High-Speed Pelleting Kit).
Materials
Expression system: E. coli BL21 (DE3) transformed with pET28a plasmid containing the gene coding for the His-tagged protein human phosphoserine phosphatase (hPSP).
Culture system: Innova S44i incubator shaker.
Centrifugation system: Harvesting Bundle (high-speed Centrifuge CR30NX plus 6 L Rotor R9A2 and 4 x 1.5 L triangular bottles) and High-Speed Pelleting Kit (Rotor R19A2 and 50 mL conical tubes). Alternatively, the Centrifuge CR22N can be used.
Methods
Bacteria proliferation and induction: 50 µL of E. coli (with pET28a) in glycerol were added to 50 mL of medium in a 250 mL flask. The culture was incubated in the Innova S44i (300 rpm, 37 °C) overnight until an OD600 of 15 – 25. 5 mL of starter culture were added to three 2.5 L bottles, each filled with 1 liter of medium. They were incubated in the S44i (250 rpm, 37 °C) until an OD600 of approx. 2 (~4 – 5 h). After adding 10 mL of IPTG the incubation was continued (250 rpm, 37 °C) for 3 h. At the end of the induction the suspension was transferred in two 1.5 L triangular bottles and spun down in the Centrifuge CR30NX with Rotor R9A2 (4,000 rpm, 30 min, 4 °C). The supernatants were discarded.
Lysis: Per gram bacteria pellet 20 mL of lysis buffer were added and resuspended. After stirring for 30 min the suspension was sonicated. The lysate was clarified by centrifugation in 50 mL conical tubes (Rotor 19A2, 12,500 rpm, 1 h, 4 °C). The supernatant was recovered and filtered through a 0.2 µm filter.
Purification by affinity chromatography: The clarified lysate (400 mL) was applied on a washed and equilibrated HisTrap™ FF crude column. After washing, elution buffer at 20 % Imidazole was used to collect the first 10 fractions. Further 10 fractions were collected using elution buffer at 100 % Imidazole.
Analysis: The absorbance at 280 nm was measured for all protein fractions to determine the amount of total protein. Samples were separated via SDS-PAGE and stained with Coomassie® blue and the enzyme activity was measured using a Malachite green phosphate assay.
Results and discussion
The elution buffer at 20 % Imidazole (first 10 fractions) was intended to remove impurities. Subsequent elution with a buffer at 100 % Imidazole was used to recover the purified protein. To identify the relevant fractions, their absorbance at 280 nm was measured.
Fig. 1 shows the results of the absorbance measurement. The elution buffer based on 100 % Imidazole dissolves a large portion of the proteins from the column with the highest amount in fraction 12.
In this Application Note*, the production of a recombinant protein is presented using a workflow for culturing and harvesting bacteria and purifying proteins. The aim is to demonstrate that a high yield of functional recombinant protein can be realized by using the Innova S44i shaker and a high-speed centrifuge (as a system solution consisting of Harvesting Bundle combined with the High-Speed Pelleting Kit).
Materials and methods
Materials
Expression system: E. coli BL21 (DE3) transformed with pET28a plasmid containing the gene coding for the His-tagged protein human phosphoserine phosphatase (hPSP).
Culture system: Innova S44i incubator shaker.
Centrifugation system: Harvesting Bundle (high-speed Centrifuge CR30NX plus 6 L Rotor R9A2 and 4 x 1.5 L triangular bottles) and High-Speed Pelleting Kit (Rotor R19A2 and 50 mL conical tubes). Alternatively, the Centrifuge CR22N can be used.
Methods
Bacteria proliferation and induction: 50 µL of E. coli (with pET28a) in glycerol were added to 50 mL of medium in a 250 mL flask. The culture was incubated in the Innova S44i (300 rpm, 37 °C) overnight until an OD600 of 15 – 25. 5 mL of starter culture were added to three 2.5 L bottles, each filled with 1 liter of medium. They were incubated in the S44i (250 rpm, 37 °C) until an OD600 of approx. 2 (~4 – 5 h). After adding 10 mL of IPTG the incubation was continued (250 rpm, 37 °C) for 3 h. At the end of the induction the suspension was transferred in two 1.5 L triangular bottles and spun down in the Centrifuge CR30NX with Rotor R9A2 (4,000 rpm, 30 min, 4 °C). The supernatants were discarded.
Lysis: Per gram bacteria pellet 20 mL of lysis buffer were added and resuspended. After stirring for 30 min the suspension was sonicated. The lysate was clarified by centrifugation in 50 mL conical tubes (Rotor 19A2, 12,500 rpm, 1 h, 4 °C). The supernatant was recovered and filtered through a 0.2 µm filter.
Purification by affinity chromatography: The clarified lysate (400 mL) was applied on a washed and equilibrated HisTrap™ FF crude column. After washing, elution buffer at 20 % Imidazole was used to collect the first 10 fractions. Further 10 fractions were collected using elution buffer at 100 % Imidazole.
Analysis: The absorbance at 280 nm was measured for all protein fractions to determine the amount of total protein. Samples were separated via SDS-PAGE and stained with Coomassie® blue and the enzyme activity was measured using a Malachite green phosphate assay.
Results and discussion
The elution buffer at 20 % Imidazole (first 10 fractions) was intended to remove impurities. Subsequent elution with a buffer at 100 % Imidazole was used to recover the purified protein. To identify the relevant fractions, their absorbance at 280 nm was measured.
Fig. 1 shows the results of the absorbance measurement. The elution buffer based on 100 % Imidazole dissolves a large portion of the proteins from the column with the highest amount in fraction 12.
Read more
Read less
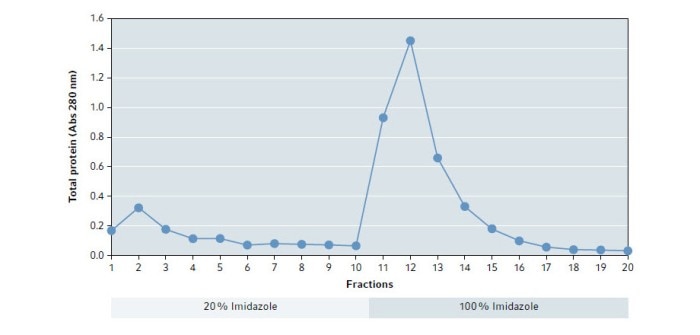
Fig. 1: Absorbance at 280 nm of eluted fractions
To determine whether and in which amount the target protein hPSP is present in the individual fractions and whether it is functional, the fractions were separated by SDS PAGE and a Malachite green phosphate assay was performed to measure the enzyme activity.
The SDS gel stained with Coomassie blue confirms that a large amount of protein was solubilized starting from fraction 11 (Fig. 2). The position of the band showing the highest density indicates that it is the target protein hPSP, which has a size of 25 kDa.
The SDS gel stained with Coomassie blue confirms that a large amount of protein was solubilized starting from fraction 11 (Fig. 2). The position of the band showing the highest density indicates that it is the target protein hPSP, which has a size of 25 kDa.
Read more
Read less
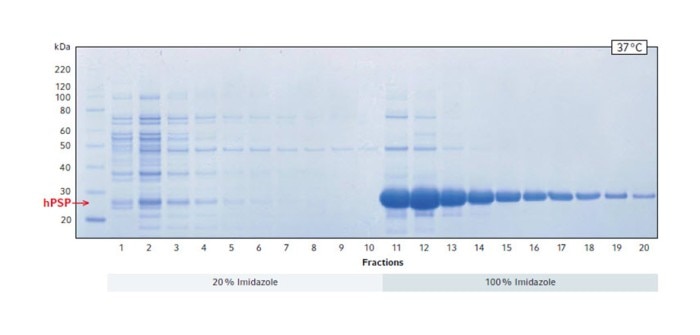
Fig. 2: Coomassie blue staining of SDS PAGE showing the total protein elution profile
The result of the assay shows that a large amount of functional hPSP was leached out by the elution buffer based on 100 % Imidazole, with a maximum in fraction 12 which correlates with the absorbance measurement (Fig. 3).
Read more
Read less
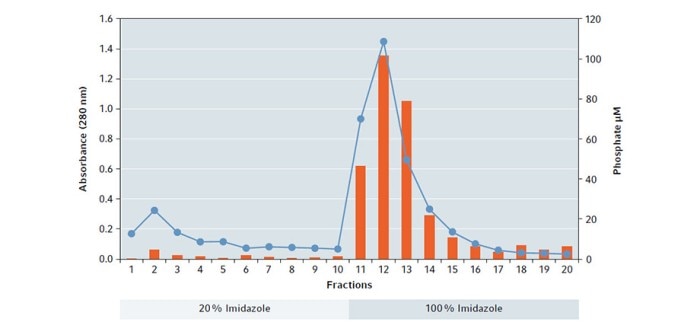
Fig. 3: Plot of absorbance measurement at 280 nm (blue dots/line) in combination of enzyme activity (orange bars) per eluted fraction
The results demonstrate that the Innova S44i and Centrifuge CR30NX, together with the appropriate accessories, can be successfully used for the production and purification of a recombinant protein. In addition to a high yield and the proven functionality of the product, the process steps could be carried out efficiently.
With its robust design and high vessel capacity, the S44i enables the culture of microorganisms at high speeds and heavy loads [2]. The Harvesting Bundle of the high-speed Centrifuge, consisting of the 4 x 1.5 L Rotor and corresponding 1.5 L bottles represents a matching system for harvesting cells. The innovative triangular format of the bottles makes handling very easy. In addition, processing 1.5 liters of culture volume per bottle, saves time during each handling step (up to 32 % of the process time compared to handling 6 bottles of 1 L volume each [1]). The bundle only needs to be completed with the High-Speed Pelleting Kit for conical tubes to cover all centrifugation steps for the production of a recombinant protein from harvest to concentration.
*Download full Application Note 451
Literature
[1] Tacheny A. Unique 4 x 1.5 L Capacity Rotor for High-Speed Centrifuges CR22N and CR30NX. Eppendorf White Paper No. 64
[2] Hartmann I., Jarvis J. The New Eppendorf X-Drive – Maximum Performance, Flexibility and Longevity for Demanding Shaker Tasks. Eppendorf White Paper No. 47
Note: Eppendorf SE reserves the right to modify its products and services at any time. This Application Note is subject to change without notice. Although prepared to ensure accuracy, Eppendorf SE assumes no liability for errors, or for any damages resulting from the application or use of this information. Viewing Application Notes alone cannot as such provide for or replace reading and respecting the current version of the operating manual.
With its robust design and high vessel capacity, the S44i enables the culture of microorganisms at high speeds and heavy loads [2]. The Harvesting Bundle of the high-speed Centrifuge, consisting of the 4 x 1.5 L Rotor and corresponding 1.5 L bottles represents a matching system for harvesting cells. The innovative triangular format of the bottles makes handling very easy. In addition, processing 1.5 liters of culture volume per bottle, saves time during each handling step (up to 32 % of the process time compared to handling 6 bottles of 1 L volume each [1]). The bundle only needs to be completed with the High-Speed Pelleting Kit for conical tubes to cover all centrifugation steps for the production of a recombinant protein from harvest to concentration.
*Download full Application Note 451
Literature
[1] Tacheny A. Unique 4 x 1.5 L Capacity Rotor for High-Speed Centrifuges CR22N and CR30NX. Eppendorf White Paper No. 64
[2] Hartmann I., Jarvis J. The New Eppendorf X-Drive – Maximum Performance, Flexibility and Longevity for Demanding Shaker Tasks. Eppendorf White Paper No. 47
Note: Eppendorf SE reserves the right to modify its products and services at any time. This Application Note is subject to change without notice. Although prepared to ensure accuracy, Eppendorf SE assumes no liability for errors, or for any damages resulting from the application or use of this information. Viewing Application Notes alone cannot as such provide for or replace reading and respecting the current version of the operating manual.
Read more
Read less
Related documents
Read more
Read less
